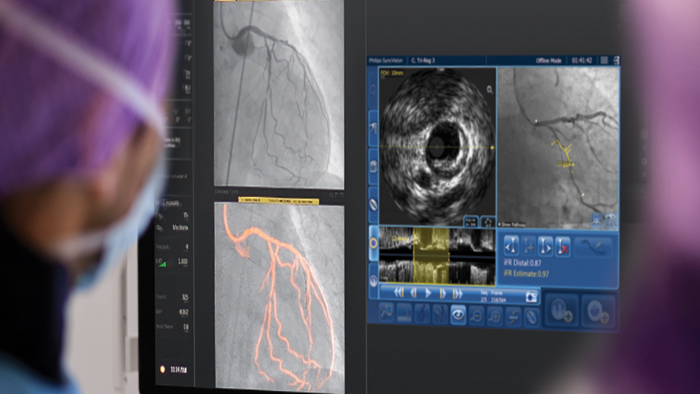
Personalise the treatment path for every patient
Our leading coronary imaging, physiology and therapy devices enable health care providers to optimise and streamline percutaneous coronary interventions (PCIs). Decide, guide, treat and confirm the right therapy for each patient at the point of care using our integrated solutions. Here you can view available coronary products, related technologies, and a compatibility chart with Philips systems
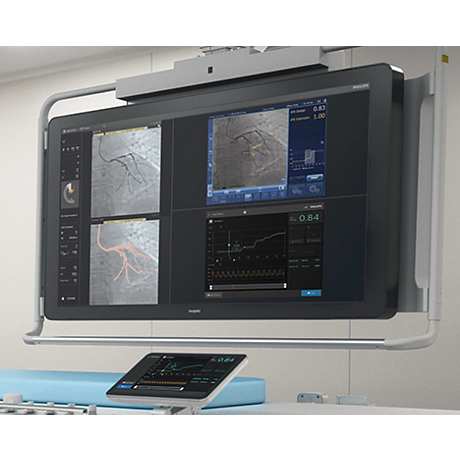
How it all fits together
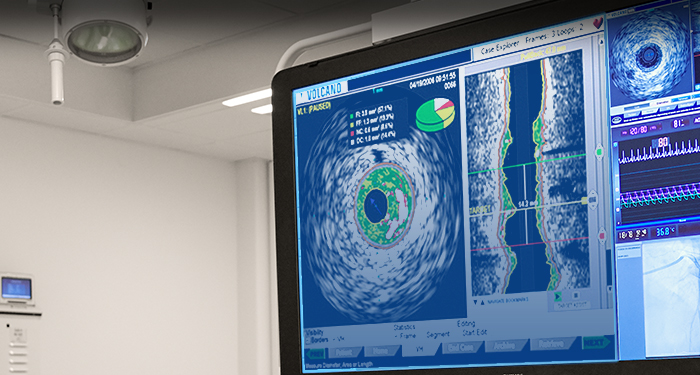
Advanced imaging systems
Philips advanced imaging systems provide a multi-modality approach with diverse capabilities for precise and accurate guidance every time. Used in conjunction with Philips catheters and atherectomy devices, you will be able to provide superior care to your patients and attain superior outcomes.
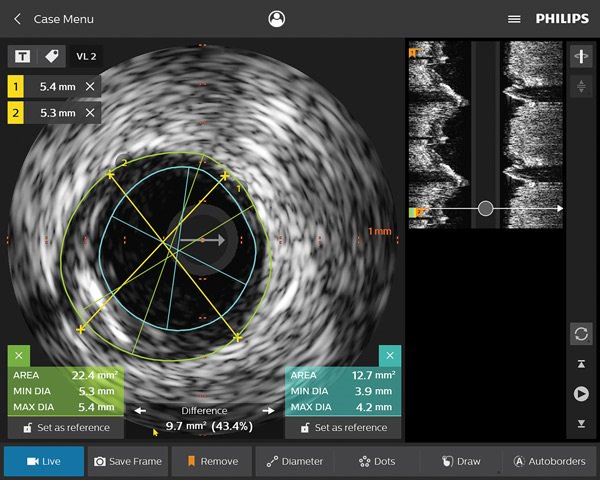
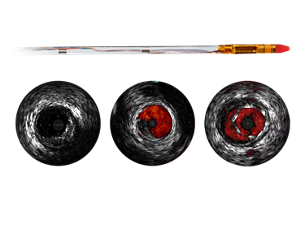
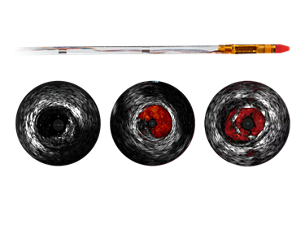
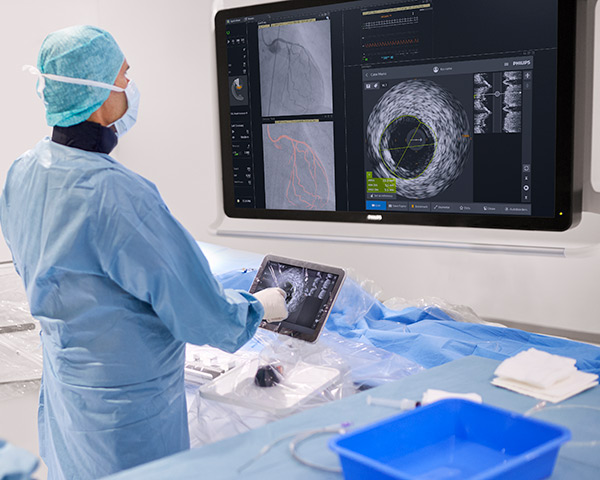
Coronary imaging catheters
Our coronary imaging catheters help assess the location of the disease and lesion morphology, including calcium and thrombus. IVUS can also be used to appropriately size stents and confirm completeness of treatment.
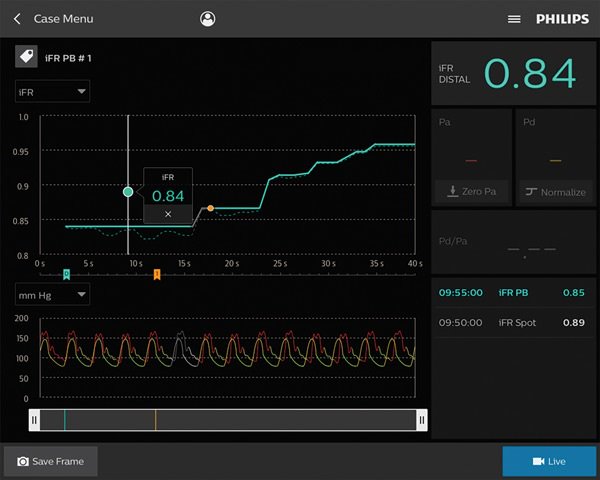
Coronary pressure and flow wires
Our coronary pressure and flow wires can measure arterial stenosis precisely and accurately.
Coronary atherectomy
Our coronary atherectomy device helps you cross, prepare and treat the most difficult coronary lesions with laser guided precision.
Specialty balloons
Our AngioSculpt PTCA scoring balloon catheter provides smooth transitions for improved crossing, elevating procedural confidence.
Coronary crossing devices
Our Quick-Cross product line helps navigate tortuous anatomy and cross vessel occlusions, allowing for exchange of guidewires, while providing a conduit for delivery of saline and diagnostic contrast agents.
Coronary
-
Advanced Imaging Systems
-
SyncVision
- iFR Co-registration
- IVUS Co-registration
- iFR and IVUS Tri-Registration
- Calibrated QCA
- Vessel enhancement
-
Coronary Imaging Catheters
-
Eagle Eye Platinum
- #1 choice of physicians for intravascular imaging (in the US).*
- GlyDx hydrophilic coating for increased lubricity
- A long, rapid exchange lumen for improved pushability
- Three radiopaque markers
- Compatibility with SyncVision for co-registration with angiography
-
Eagle Eye Platinum ST
- A 2.5 mm tip-to-imaging distance designed to assess more of the vessel than standard catheters
- Plug-and-play simplicity
- Three radiopaque markers
- GlyDx hydrophilic coating
- SyncVision compatibility
Compatibility with Philips systems and technologies
 |  |  |  |
Coronary imaging catheters | |||
|---|---|---|---|
|
|
|
|
Coronary pressure and flow wires | |||
|
|
|
|
Coronary atherectomy | |||
|
|
|
|
Technologies | |||
|
|
|
|
1. Not all IVUS catheters include ChromaFlo imaging capability. 2. FFR, iFR, and iFR Scout are compatible with Philips pressure and flow wires. 3. SyncVision requires an IntraSight, Core, Core Mobile system. Not all catheters are compatible with SyncVision. 4. Specialty Balloons and Crossing Devices do not require capital equipment, hence they're not listed in this compatibility chart. 5. Co-registration tools available within IntraSight 7 configuration via SyncVision. *Safety and effectiveness of VH IVUS for use in the characterization of vascular lesions and tissue types has not been established.
Family man brought back to his everyday life
Ian is a father of three living in London and loves his active Sunday afternoons with his family. One day while shopping with his children, he experienced loss of breath and had trouble keeping up with his children, so he sought the help of Dr. Al-Iamee.
Related technologies
-
Only Philips iFR Co-registration provides advanced physiologic guidance during PCI procedures to help you determine where and how to treat for precise, patient-focused care.
Click here to read more -
![Coronary IVUS]()
Decide, guide, and confirm the right treatment and PCI strategy for each patient with Philips Intravascular ultrasound (IVUS) coronary catheters.
Click here to read more -
![Advanced IVUS Imaging Technology]()
Learn how Philips can help streamline your procedures with ChromaFlo imaging, IVUS Co-registration and iFR and IVUS Tri-registration.
Click here to read more -
![VH IVUS]()
VH IVUS imaging provides a colorized tissue map of plaque composition with automated lumen & vessel measurements.
Click here to read more
Supporting you at every turn

Customer Support
Connect with our customer service team if you have questions or issues with your Philips software and products.

Clinical Informatics
Information for clinicians, hospitals, and general healthcare professionals on the subjects of coding and payment for Philips IGT Devices technologies.

Instructions for Use
Instructions for Use (IFUs) of our products are available online for your convenience.

Customer Sales
Have a sales representative contact you, or request product literature.